|
|
Original Article
| ||||||
| Do small for gestational age fetuses' exhibit circadian changes in fetal heart rate parameters as do appropriate for gestational age fetuses? | ||||||
| Habiba Kapaya1, Emma R. Dimelow2, Dilly Anumba3 | ||||||
|
1Clinical Lecturer (NIHR), Department of Oncology and Metabolism, Academic Unit of Reproductive & Developmental Medicine, 4th Floor Jessop Wing, Tree Root Walk, Sheffield S102SF, UK, the University of Sheffield.
24th year medical student, The University of Sheffield. 3Consultant in Obstetrics & Gynaecology, Department of Oncology andMetabolism, Academic Unit of Reproductive & Developmental Medicine, 4th Floor Jessop Wing Tree Root Walk, Sheffield S102SF, UK, the University of Sheffield. | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] |
| How to cite this article |
| Kapaya H, Dimelow ER, Anumba D. Do small for gestational age fetuses' exhibit circadian changes in fetal heart rate parameters as do appropriate for gestational age fetuses? Edorium J Matern Child Health 2017;2:1–8. |
|
Abstract
|
|
Aims:
Circadian changes in basal fetal heart rate (FHR) and fetal heart rate variation are observed in appropriate for gestational age (AGA) fetuses. Small for gestational age (SGA) fetuses are at greater risk of complications and may not demonstrate similar changes. This study was undertaken to investigate circadian changes in FHR in SGA fetuses in ambulatory patients in home environment using a portable fetal electrocardiogram recording device.
Methods: Prospective study on 31 singleton pregnancy >24 weeks gestation, no evidence of fetal malformation and an estimated fetal weight below tenth gestational centile on ultrasound scan. Fetal heart rate recordings were collected up to 17 h, averaged over 90 min intervals, and compared between day and night data from the same individual. Results: Basal FHR reduced at night-time (p<0.001) and with gestation (p=0.013), whereas FHR variation remained unchanged (p >0.5). Fetal gender did not influence variation in day:night FHR parameters. Conclusion: Unlike AGA fetuses, SGA fetuses demonstrated significant circadian changes only in basal FHR. The day-night difference in basal FHR was more pronounced with advancing gestation, implying a significant fetal role in controlling the circadian pattern. In contrast to AGA fetuses, FHR-variability in SGA fetuses did not increase with gestation, suggesting under-development of fetal autonomic nervous system. Clinicians need to consider these differences when interpreting FHR data in these two clinical settings. | |
|
Keywords:
Circadian, Fetal heart rate, Fetal electrocardiogram, Pregnancy
| |
|
Introduction
| ||||||
|
It is well established that small for gestational age (SGA) fetuses are at higher risk of perinatal morbidity and mortality compared to appropriate for gestational age (AGA) fetuses [1] [2] [3] . Consequently much work has investigated approaches to antenatal monitoring of SGA fetuses including assessment of electronic fetal heart rate (FHR) monitoring [4]. Adverse cardiovascular and neurological outcomes have been reported in SGA infants in the early neonatal period [2] [5]. There is growing evidence that children born SGA have impaired autonomic nervous system (ANS) function which contributes to impaired cardiovascular rhythmicity [6]. Clinical studies have provided strong evidence of an association between circadian rhythmicity and several disorders of the cardiovascular system [2]. From as early as 20–22 weeks to the end of pregnancy circadian changes have been described in AGA fetuses during various physiological activities. These include FHR and variability, FHR accelerations and fetal movements [7] [8] [9]. In addition, studies have also shown a direct relationship between maternal and fetal heart rates at various stages in pregnancy, suggesting that fetus is not autonomous in this respect [9] [10]. Few studies have examined whether SGA fetuses exhibit similar circadian variation in FHR patterns as AGA fetuses. In a previous report we employed a small, portable fetal electrocardiogram device (Monica AN24) to demonstrate circadian variation in FHR patterns in AGA fetuses whilst the women continued their-day-today activities [11]. We hypothesized that SGA fetus may exhibit altered circadian changes in FHR parameters compared to AGA fetuses. To evaluate our hypothesis, we analyzed 17 h ambulatory FHR data in a cohort of otherwise healthy pregnant women carrying SGA fetus in their home environment. | ||||||
|
Materials and Methods
| ||||||
|
A prospective, non-randomized observational study approved by the North-West Preston NRES Research Ethics committee (15/NW/0278) was conducted at Jessop Wing Hospital in Sheffield, UK. The study group consisted of 31 women with singleton pregnancies between 24 and 40 weeks gestation and ultrasound-estimated fetal weight (EFW) less than 10th gestation centile [12]. Women recruited for the study were normotensive and suffered no pre-existing medical conditions. At the time of recording, women who smoked were advised to press an "event button" on the monitor, every time they smoked. For the purpose of analysis, all those 30 minutes epochs where the "event mark" for smoking occurred were excluded. None of the women drank alcohol or received no other medication at the time of evaluation. Patients with any fetal malformations, who were in active labor, and who had multiple pregnancies were excluded from the study. After giving informed written consent, the fetal ECG monitor was fitted by placing five skin electrodes in standardized positions on the maternal abdomen [13]. When fully charged the device is capable of obtaining recordings lasting up to 20 hours [14]. Participants were allowed to go home and carry on with daily activities whilst wearing the monitor. They were advised to turn off and remove the monitor after 20 hours had elapsed. However, they were at liberty to take off the monitor at any time if they experienced any discomfort. The monitor was either collected from patient's home by the research team or returned by the patient after the monitoring session. Subsequently, the participants filled in a questionnaire to record their views and experience of wearing the device, and to express if they were willing to wear the device again. The electrophysiological signal recorded within the monitor calculated beat-to-beat information on FHR in addition to the average FHR data obtained from Doppler-based CTG monitors. It also recorded the FHR parameters generated by computerized analysis developed by Dawes and Redman [15] [16]. In order to investigate changes in FHR pattern between the day and night times, three consecutive 30 minutes frames (90 minutes data) with FHR acquisition success rate of =80% were selected during the "day" and "night" periods for each subject. The three selected 30 min epochs were averaged and compared between the day and night recordings of the same individual. The following FHR parameters derived by the Dawes Redman analysis were recorded and evaluated:
Statistical analysis | ||||||
|
Results | ||||||
|
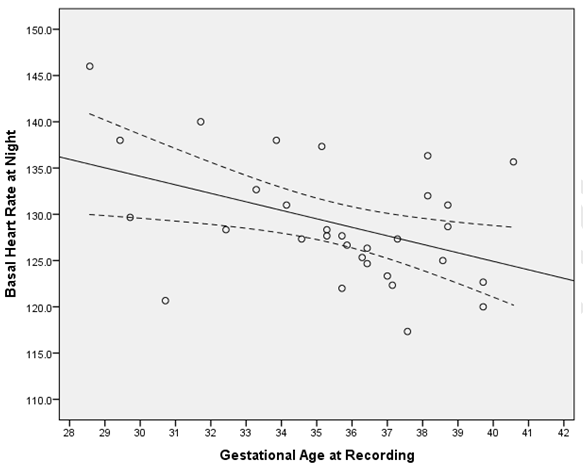
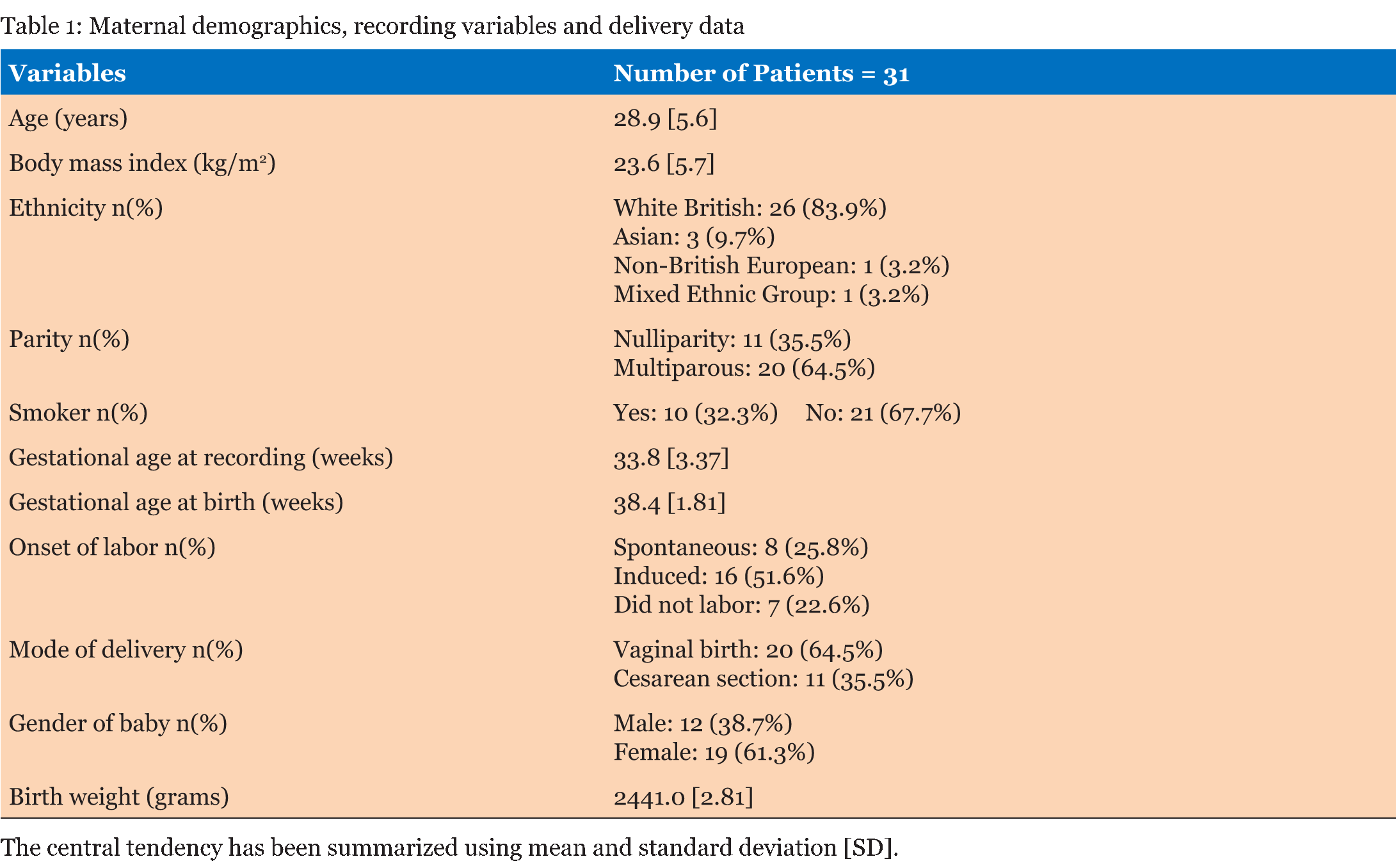
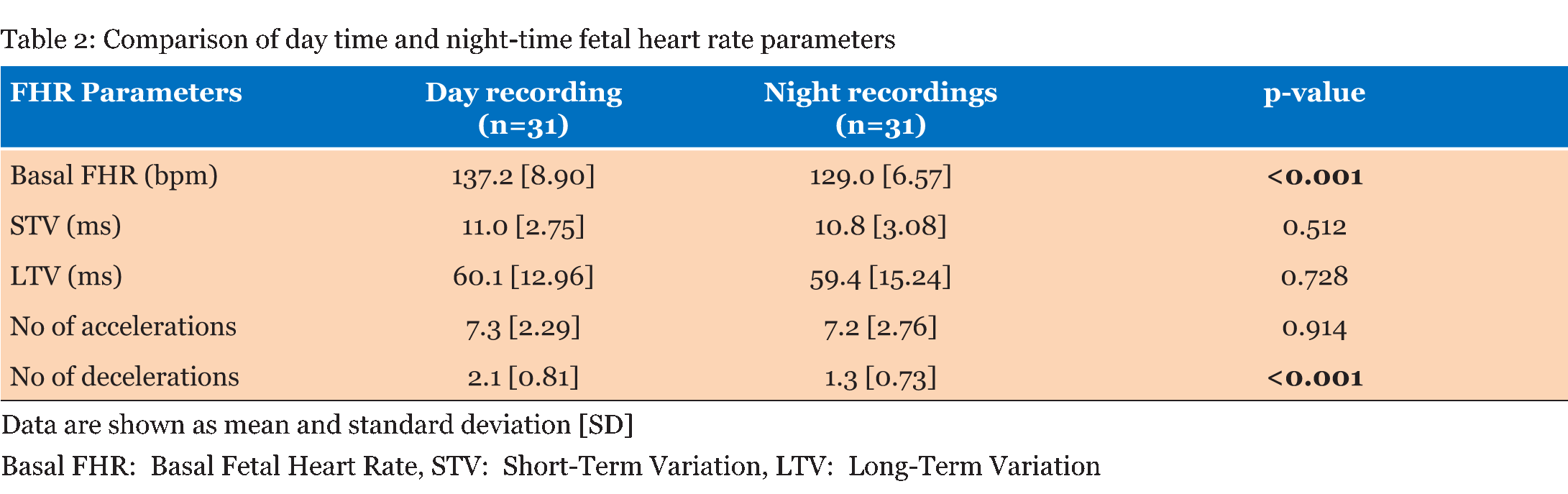
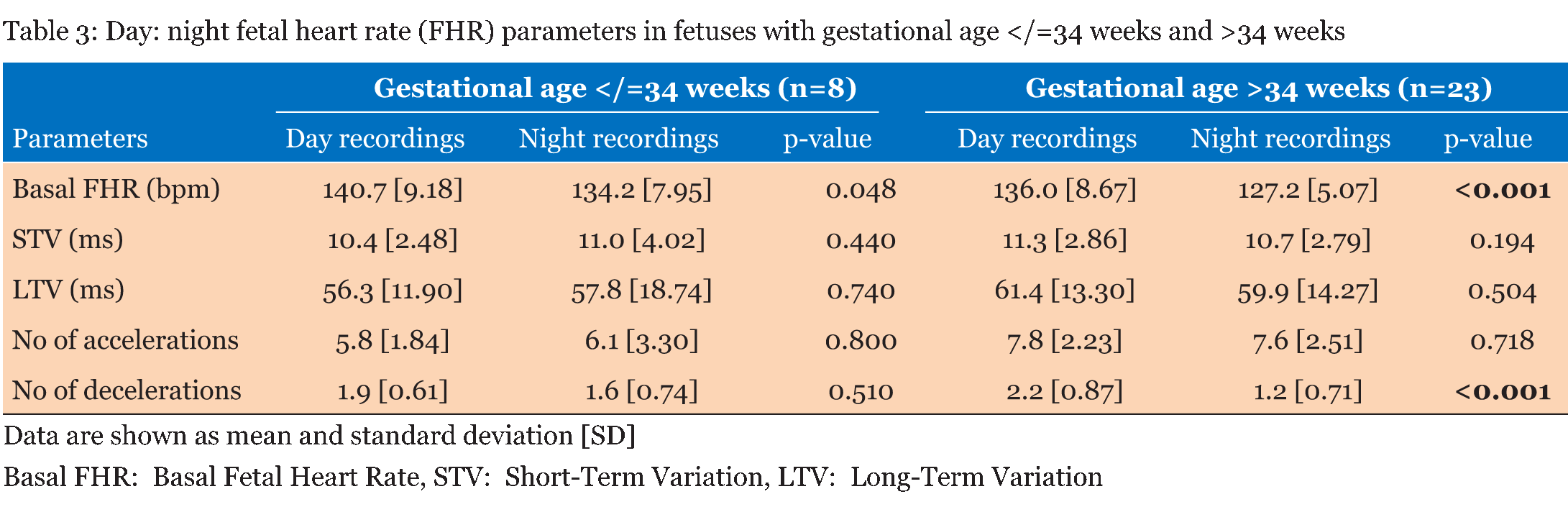
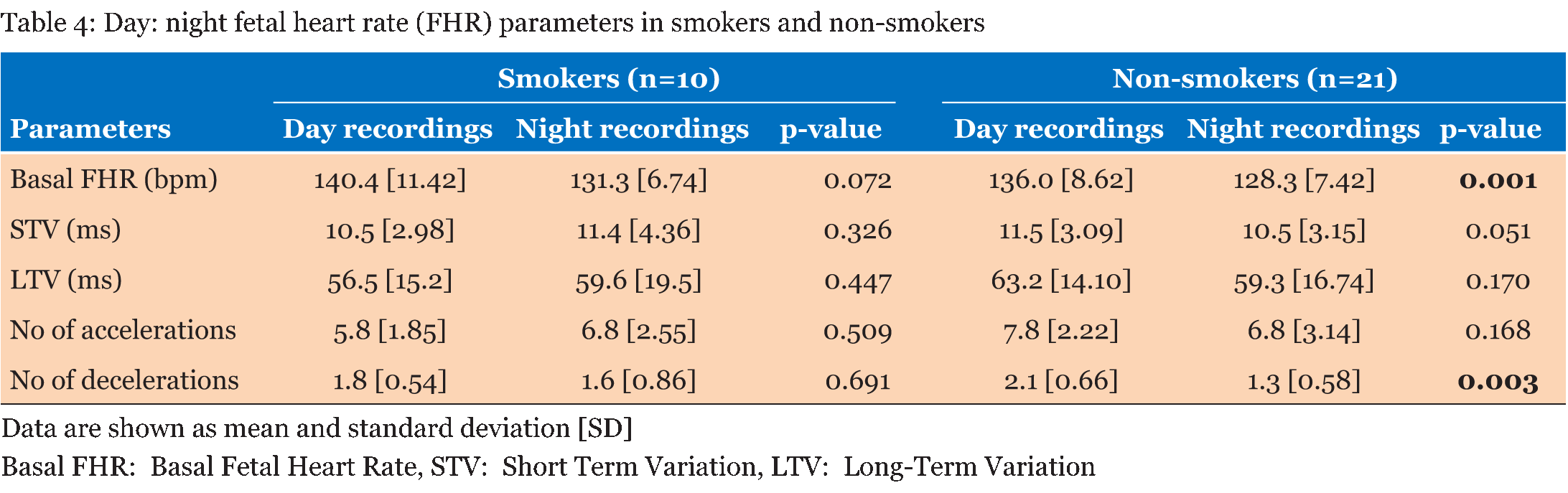
Table 1 summarizes the basic demographic data for the women, study recording and delivery outcome variables. 62 recordings (31 days and 31 nights) made over 90 minute epochs were analyzed from 31 women recruited at home (Table 2). Significant differences were observed in the basal FHR and number of decelerations (p <0.001), both decreased at night time. However, STV, LTV and number of accelerations did not show significant variations between the day and night recordings (p>0.5). Further analysis during the night-time, when external stimuli were reduced, showed the change in basal FHR to be strongly inversely correlated with gestational age; p=0.013 (Figure 1). However, other FHR parameters did not show a significant correlation with gestational age, (p>0.1). Since there was a clear effect of gestational age on basal FHR, the data were then subdivided into two groups. Table 3 demonstrates that fetuses below 34 weeks of gestation did not exhibit similar circadian changes in FHR patterns as mature fetuses did. In our study, we had 10 women who smoked during the course of recording. Although, we excluded all the 30 minutes epochs where smoking occurred, we examined day: night changes in FHR parameters separately between smokers and non-smokers to assess whether maternal smoking status disrupts circadian changes in FHR pattern. Results given in Table 4 clearly indicates that compared to fetuses of non-smoking mothers, fetuses of smoking mothers did not show significant circadian variation in any of the FHR parameters. We did not observe any gender-related difference between 12 males and 19 females fetuses during the day and night time; p>0.1 Table 5. Of the 31 women studied, 25 gave birth to babies with a birth weight below the tenth percentile. | ||||||
|
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
|
| ||||||
| ||||||
|
Discussion
| ||||||
|
This study has demonstrated that there is a clear circadian pattern in the FHR in SGA fetuses: there was a greater influence of time of day on basal FHR and number of decelerations, both of which reduced significantly at night time (p<0.001). However, unlike AGA fetuses [11], we did not observe significant circadian changes in FHR variation or number of acceleration in SGA fetuses. In addition, we did not find any difference in the day and night recordings between male and female fetuses as observed in AGA fetuses [11]. Maternal activity, diet intake, psychological and physiological conditions can influence FHR [17][18][19]. The fact that information regarding these maternal factors was not collected can be considered as a limitation of this study. However, the ability to achieve good quality recordings of the FHR over prolonged periods (up to 17 hours) in SGA fetuses whilst the mother continued with normal activities at home is a novel approach, permitting the assessment of the FHR under normal (uncontrolled) conditions. This is the first study to report changes seen in the FHR in relation to time of day in SGA pregnancies where maternal mobility was not restricted. It is not possible to comment on whether the control of the circadian pattern in the fetus is influenced by maternal activity because we did not investigate the relationship of the FHR to that of the mother. However, the fact that advancing gestational age has an influence on the magnitude of the day:night difference in basal FHR supports the notion that there a major fetal component to control of the circadian rhythm of FHR in SGA fetuses. Several studies have shown blunting circadian rhythm in pregnancies complicated by pre-eclampsia [20][21]. Disruption of circadian rhythmicity in women with pre-eclampsia may result in hampered development of fetal circadian rhythmicity. However, for the purpose of this study we excluded women with SGA fetuses who had pre-eclampsia. In addition, during evaluation, no woman received any medication that could have influenced FHR and FHR variability [22] [23] [24]. However, ten women smoked while they were monitored. Although, we excluded all 30 minutes epochs in the recordings where the "event marker" for smoking was pressed by the woman, our study was limited by the fact that we relied on women to provide information on smoking rather than making an objective assessment of tobacco exposure. Nicotine consumption has not only been shown to produce greater effect in women but also delays the diurnal peak of circadian heart rate pattern [25]. This may explain why fetuses of smoking mothers did not exhibit circadian changes for any of the FHR parameter. Another limitation of the study was the inability to standardize the day recordings. This limitation was related to the success rate of the recording. The average overall success rate was 48.6%, which is lower than reported in previous studies [13] [16]. Graatsma et al. [13] reported 82% of their recordings had a signal quality of >60%. However, their study included fetuses of all sizes and was not limited to fetuses with estimated birth weights below the tenth percentile. Additionally, Graatsma et al. [13] standardized their recording time from 5 pm until 8 am, which meant that the majority of the recordings were made during resting hours, and therefore, fewer recordings were disturbed by maternal movements. In our study, the device was fitted after the clinic appointment, which meant that recording time started between 9 am and 5 pm. As a result, the amount of time at rest varied significantly between the recordings. A lower success rate meant that it was not possible to fully standardize the daytime recordings, therefore we randomly selected the best 90 min FHR data with good fetal signals at daytime, implying that our study may have suffered selection bias. Nonetheless, selection of 90 min FHR data guaranteed the analysis of high variation episodes as in the human fetus the cycle of active and quiet sleep ranges from 60–90 minutes [25]. However, a higher success rate at night (84.1%) meant that it was possible to fully standardize all recordings at night time. Fetal heart rate patterns change markedly with gestation, with a decrease in basal FHR and an increase in FHR variability as pregnancy advances, reflecting maturation of the fetal autonomic nervous system and development of fetal behavioral pattern in AGA fetuses [26] [27]. Although, SGA fetuses exhibited reduction in basal FHR with advancing gestation and at nighttime, they did not show any change in FHR variation as observed in our previous work [11] and the work published by others on AGA fetuses [7] [9]. This finding can be interpreted as a reflection of immaturity of the cardiovascular autonomic control system in SGA fetuses and is consistent with the current knowledge that SGA children display significantly altered cardiovascular circadian rhythmicity [2] [6], and an impaired and an immature autonomic cardiovascular control, relating to an increased risk of cardiovascular disease [1] [5][27]. There is evidence that the development of cardiovascular disease is initiated by unfavorable conditions during intra-uterine life, implying that the cardiac autonomic balance may be permanently altered according to the concept of fetal programming [28]. The correlation of heart rate and variability with gender remains a matter of interest to many scientists. Previous studies that have examined the influence of gender on FHR variability in AGA fetuses have reported conflicting results. Lange et al. [29] suggested that gender does not need to be considered when assessing FHR variability, whereas Fleisher et al. [30] and Amorim-Costa et al. [31] reported significant gender-related differences in FHR parameters at various gestational ages. With regard to SGA pregnancies, there is insufficient data on the influence of gender on FHR pattern. Kwon et al. [4] found significant gender-related differences in the FHR variability in a study of 14 males and 15 females SGA fetuses during the last two-hour of labor preceding delivery. Our results differ from them, as we did not find any significant difference in FHR pattern between the SGA male and female fetuses. This discrepancy can be explained by experimental setting and sample size. We examined FHR during the antenatal period whilst Kwon et al. carried out studies during labor [4]. Secondly, we did not have equal distribution of male and female fetuses in our study cohort. | ||||||
|
Conclusion
| ||||||
|
This study confirms that small for gestational age (SGA) fetuses demonstrate a clear circadian pattern in underlying basal fetal heart rate (FHR) and decelerations, both of which reduce significantly at nighttime. An analysis of these fluctuations is useful in the evaluation of the health of the fetus. In general clinical settings, FHR is usually monitored in the daytime. As the number of decelerations is higher in the daytime, we conclude that evaluation of fetal wellbeing is insufficient if done only during the day. Larger studies will be required to determine whether lack of circadian variation in FHR patterns in SGA fetuses correlates with adverse pregnancy indices and outcomes such as fetal acidosis. Nevertheless clinicians should take cognizance of these changes and exercise caution when applying and interpreting FHR tests for antenatal fetal wellbeing. | ||||||
|
Acknowledgements
| ||||||
|
This study was funded by Jessop Wing Small Grant Scheme. We gratefully acknowledge Monica Healthcare, Nottingham, UK for providing electrodes in kind for the study. | ||||||
|
List of Abbreviations
| ||||||
|
AGA Appropriate for gestational age | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions:
Habiba Kapaya – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Emma Dimelow – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Dilly Anumba – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© 2017 Kapaya H et al et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|